Looking for more information?
Contact Biocair to discuss your requirements for COVID-19 vaccine supply chain management.
White Paper
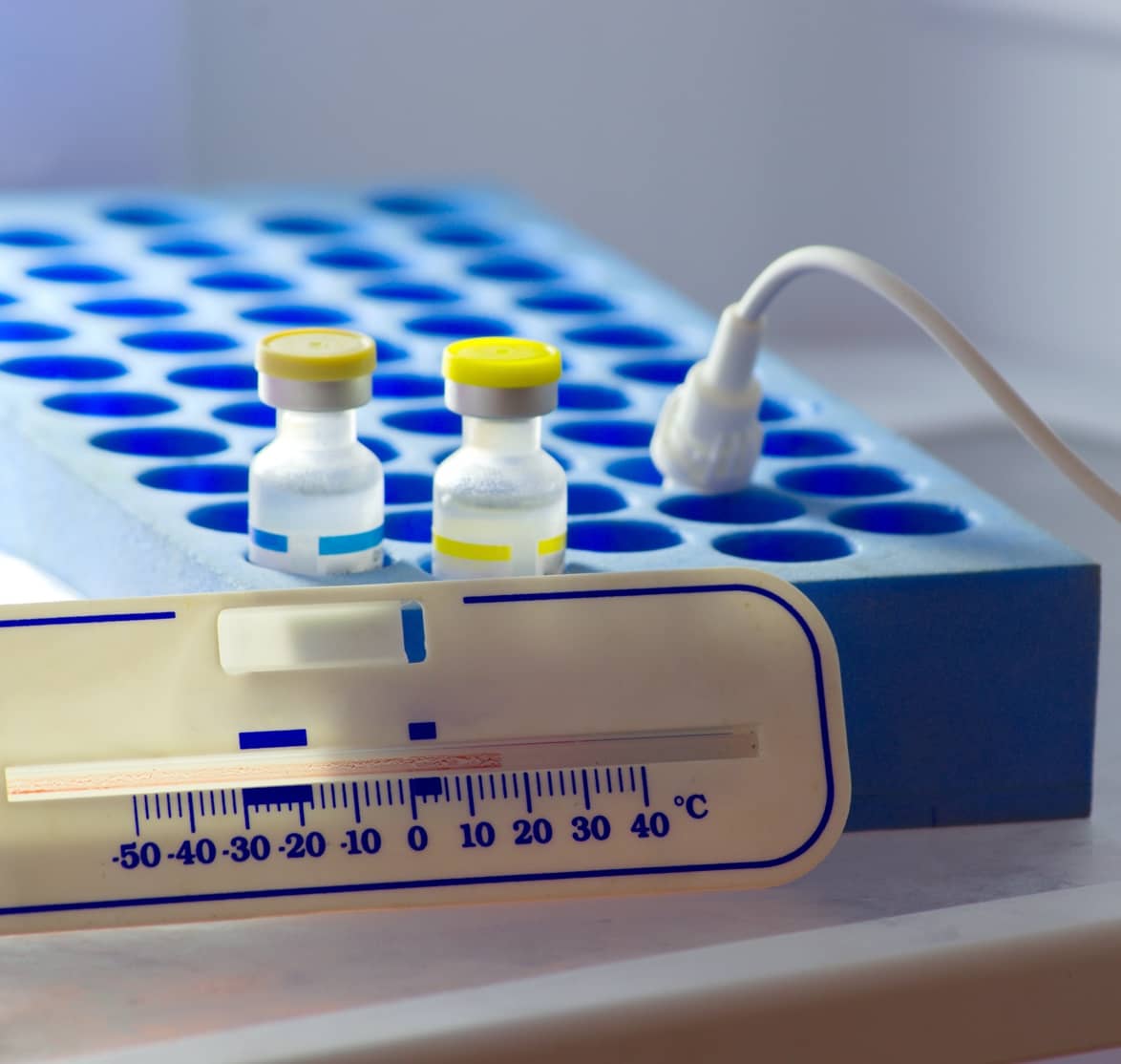
As the pharmaceutical sector cannot function without efficient, flexible and secure cold chains, it will require increasingly sophisticated techniques and technologies to deliver global vaccination programs and industry-leading logistical services.
Vaccines present several cold chain challenges, as they must be delivered within a narrow temperature range to the end-user to ensure their potency. Ensuring these medicines are effective means understanding the potential impact of temperature and environment.
Of the top 50 drugs today, half need a cold supply chain to support them. As biopharma products accelerate, supply chain service providers will have to expand and diversify services to meet the increasing demand.
Temperature control is more than maintaining low temperatures, with cold chain logistics both presenting unique challenges. Logistical service suppliers need to offer controlled ambient, refrigerated, frozen, deep frozen and cryo frozen services – each with their own temperature profile and application.

PwC in their report 'Pharma 2020: Supplying the future’ concluded: “Increasing demand for biologics has stimulated the development of specialist logistics providers capable of handling very sensitive pharmaceutical freight. Many provide specialized service where each shipment is transported in temperature – and humidity-controlled – conditions, monitored from a dedicated call center using web-based tracking and reporting, and delivered directly to the customer's door.”
According to Pharmaceutical Commerce, the spending on cold chain biopharma logistics will hit USD$18 billion, most which will be spent on air and parcel cold consignment shipping.
Often, pharma products will move through several areas of transit and this is where the challenges in cold chain logistics become apparent.
Mapping the temperature of storage facilities is critical, as temperature-sensitive products must have their temperature maintained throughout transit. Cold rooms, warehouses, order assembly points and loading docks all have specific temperature profiles that must be monitored and maintained based on the pharma products being stored or transported.

Having detailed information about a vaccine shipment as it passes through the cold chain is vital to resolve any challenges its journey may present.
A risk assessment analysis reveals any weak points in the cold chain that must be improved to maintain the temperature integrity of the product being transported.
One of the most critical aspects of creating a reliable cold supply chain is ensuring that the human component has the training and knowledge they need to ensure temperature excursions are kept to a minimum. With most temperature failures caused by human error, it's vital to understand where the risk factors exist across a cold supply chain and mitigate them where possible.
As the drive to develop more innovative vaccines, drugs and gene therapies accelerates, and new biologic and biosimilar drugs come onto the market, there is a greater need for supply chain support.
Personalized medicine and therapies are also expanding. Producing CAR-T cells, for instance, require small batches that are difficult to scale, yet still, demand robust and efficient logistical support services.
Here, target medicine will need targeted supply chains. With a drugs market set to grow to USD$90 billion by 2023 (Precision Medicine Market), cold chain logistics providers will have to react.
The challenge is to create general and customized cold supply chains that can support the widening needs of pharma companies and patients. Pharma 4.0 will embrace the advanced technologies that are developing in consignment tracking, insulation and data communications.
Ultimately, cold chain logistics suppliers will become more sophisticated, building personalized services that are robust, secure, reliable and agile enough to overcome supply chain challenges.
Contact Biocair to discuss your requirements for COVID-19 vaccine supply chain management.